
Optimal Treatments for NSCLC Patients Harboring Primary or Acquired MET Amplification - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36148917/
Background : In non-small cell lung cancer (NSCLC) patients harboring MET mutations, MET-tyrosine kinase inhibitors (TKIs) have been proven to achieve a good response. However, the relative efficacy of different...
Conclusions: Immunotherapy showed a low response in patients harboring MET alterations, even those with concurrent high PD-L1 expression. MET-TKIs might be an optional treatment with worth-expecting efficacy. However, chemotherapy plus bevacizumab could benefit the subpopulation of patients harboring acquired MET amplification after the failure...

Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III non-small cell lung cancer - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36149029/
Chemoradiotherapy followed by consolidation durvalumab (CCRT+D) improves survival in patients with stage III non-small-cell lung cancer (NSCLC). We compared recurrence patterns and survival in the CCRT+D and CCRT cohorts. We...
Conclusion: Consolidation durvalumab decreased both LR and DM, and significantly improved PFS. However, in-field recurrence was still a major problem, as well as DM.

A Phase II Trial of Atezolizumab Plus Carboplatin Plus Pemetrexed Plus Bevacizumab in the Treatment of Patients with Stage IV Non-Squamous Non-Small Cell Lung Cancer: Big Ten Cancer Research Consortium (BTCRC)- LUN 17-139
Source : https://www.clinical-lung-cancer.com/article/S1525-7304(22)00157-7/fulltext
Micro-AbstractThe BTCRC-LUN 17-139 is a single arm phase II study which evaluated the combination of Atezolizumab plus Carboplatin plus Pemetrexed plus Bevacizumab in stage IV treatment naïve non-squamous NSCLC. The...
Conclusion: ABCPem was associated with increased PFS compared to historical controls but this difference did not meet the statistical significance. Three on-treatment deaths and 5 thromboembolic events prompted early closure.
-
 Albert Dekker3yrAvastin provides great symptomatic relief
Albert Dekker3yrAvastin provides great symptomatic relief

Validation of Patras Immunotherapy Score model for prediction and prognosis of patients with advanced NSCLC treated with nivolumab or pembrolizumab: results from a European multicentre study - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36105886/
This study provides further validation of PIOS in aNSCLC patients treated with anti-PD-1 monotherapy.
Conclusions: This study provides further validation of PIOS in aNSCLC patients treated with anti-PD-1 monotherapy.
-
 Albert Dekker3yrdo any guidelines in US endorse this or a different scoring system
Albert Dekker3yrdo any guidelines in US endorse this or a different scoring system
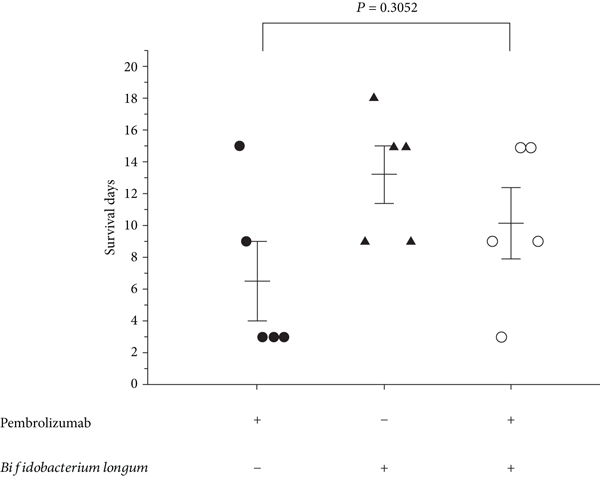
Construction of a Humanized PBMC-PDX Model to Study the Efficacy of a Bacterial Marker in Lung Cancer Immunotherapy
Source : https://www.hindawi.com/journals/dm/2022/1479246/
Construction of a Humanized PBMC-PDX Model to Study the Efficacy of a Bacterial Marker in Lung Cancer Immunotherapy: Commensal microbiome is a key factor of lung cancer immunotherapy efficacy. Elucidating...
Conclusion/Relevance: It was found that although both Bifidobacterium longum and immunotherapy drug pembrolizumab alone showed suppressing tumor growth, the efficacy of pembrolizumab was attenuated when administrated to mice colonized with Bifidobacterium longum. Further exploration revealed that Bifidobacterium longum caused significant...
